Answer:
Molecular mass of unknown gas is 87.58 grams per mol.
Step-by-step explanation:
Let x be the molecular mass of the unknown gas.
Rate of effusion of a gas and its molecular mass is related as:
r ∝
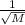
where r is rate and M is molecular mass.
Molecular mass of He gas = 4 g/mol
Given: Same amount of gas is effused and at same conditions.
Let us say V mL of gas effused.
Then rate = V/t where t is time taken for effusion.
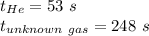
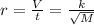
Since same amount of gases are effused V doesn't matter.
We can say:
t ∝ √M ⇒
then
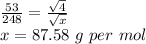
Hence molecular mass of unknown gas is 87.58 grams per mol.