Answer:

Step-by-step explanation:
First of all, we need to understand that propene,
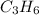 , is an alkene. This can easily be understood from the general formula of alkenes having an expression of
, is an alkene. This can easily be understood from the general formula of alkenes having an expression of
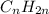 . This means when you spot a formula with twice as many hydrogen atoms as carbon atoms, you will have an alkene (or a cycloalkane, but no reaction would take place with a cycloalkane).
. This means when you spot a formula with twice as many hydrogen atoms as carbon atoms, you will have an alkene (or a cycloalkane, but no reaction would take place with a cycloalkane).
Alkenes have a double bond and they produce an addition reaction with bromine water. The evidence for this experimentally is when you mix an alkene with bromine water, the color of the solution disappears.
The two bromine atoms are joined to the two carbons forming a double bond by removing the pi bond and making a saturated molecule with single bonds only. That said, we have a simple addition reaction in which the bromine atoms are added to the molecule.