Answer:
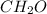 ,
,
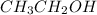
Step-by-step explanation:
Hydrocarbons are compounds which only consist of hydrogen atoms and carbon atoms. The presence of any other atom, such as oxygen, any halogen, nitrogen or sulfur, would not let us classify a compound as hydrocarbon.
Regardless of the number of atoms present in the structure, all we need to identify a hydrocarbon is just to make sure that it contains merely carbon and hydrogen atoms.
Notice that the first compound only consists of C and H atoms, so it is a hydrocarbon, just as compound 2.
The third and the fourth compounds have oxygen in them, so they are not hydrocarbons, as they don't have only C and H atoms in them.