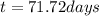Answer: 71.72 days
Step-by-step explanation:
This problem can be solved using the Radioactive Half Life Formula:
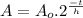 (1)
(1)
Where:
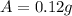 is the final amount of Iodine-131
is the final amount of Iodine-131
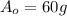 is the initial amount of Iodine-131
is the initial amount of Iodine-131
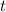 is the time elapsed
is the time elapsed
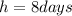 is the half life of Iodine-131
is the half life of Iodine-131
Knowing this, let's substitute the values and find
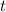 from (1):
from (1):
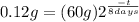 (2)
(2)
 (3)
(3)
Applying natural logarithm in both sides:
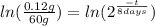 (4)
(4)
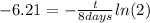 (5)
(5)
Finding
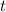 :
: