Answer:
The curve is still exponential but decreases at a lower rate for a greater half-life;
The greater the half-life, the slower radioactive decay is.
Step-by-step explanation:
From the context of the actual problem, it looks like you plotted the number of non-decayed atoms against time. Since you are analyzing a radioactive decay in this problem, the number of the atoms remaining for the first-order rate law can be represented by the following equation:
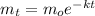
Here k is the rate constant. It is defined in terms of half-life by the following relationship:
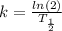
That said, in terms of half-life, our equation becomes:
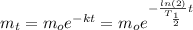
Notice that the greater the half-life is, the less negative the coefficient in front of the time variable in the exponent.
As a result, the decay for a greater half-life would occur at a lower rate. The curve would still be exponential in terms of shape but would decrease at a lower rate.
We may conclude that the greater the half-life, the slower radioactive decay is.