Answer:
Choice C.) A
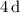 electron.
electron.
Step-by-step explanation:
In an atom, each electron has a unique set of four quantum numbers.
 is the principal quantum number. It gives the index of the energy shell that contains this electron. Hence, it must be a positive whole number (1, 2, 3, etc.) In this case,
is the principal quantum number. It gives the index of the energy shell that contains this electron. Hence, it must be a positive whole number (1, 2, 3, etc.) In this case,
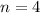 . Hence, this electron is in the fourth main energy shell. Think about this number as giving the size of the orbital that holds this electron.
. Hence, this electron is in the fourth main energy shell. Think about this number as giving the size of the orbital that holds this electron.
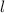 is the orbital angular momentum quantum number. It gives the type (shape) of the orbital that holds this electron.
is the orbital angular momentum quantum number. It gives the type (shape) of the orbital that holds this electron.
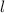 should also be a whole number. However, unlike
should also be a whole number. However, unlike
 , the value of
, the value of
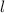 starts from
starts from
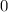 and has an upper bound,
and has an upper bound,
 . Here are the meanings for some common values of
. Here are the meanings for some common values of
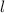 :
:
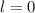 corresponds to an s-orbital. (Requires
corresponds to an s-orbital. (Requires
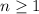 .)
.)
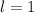 corresponds to a p-orbital. (Requires
corresponds to a p-orbital. (Requires
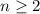 .)
.)
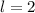 corresponds to a d-orbital. (Requires
corresponds to a d-orbital. (Requires
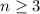 .)
.)
As a side note, in each energy level, each type of orbital can hold more than one electrons. The other two quantum numbers,
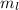 and
and
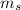 , help distinguish between these electrons. However, that's not very relevant to this problem.
, help distinguish between these electrons. However, that's not very relevant to this problem.