Answer:
116.69 g.
Step-by-step explanation:
Let's write down the reaction taking place when barium chloride reacts with sodium sulfate. This is a double displacement reaction, meaning the anions will be exchanged to produce barium sulfate and sodium chloride:
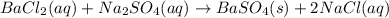
Let's find the moles of sodium sulfate. Given the volume of:
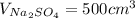
Convert this to milliliters knowing that:
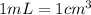
Therefore:
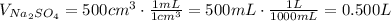
Also, we know that:
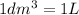
Convert the molarity into mol/L:
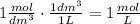
Multiply molarity by volume to find moles of sodium sulfate reacted:
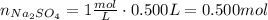
According to stoichiometry of the equation, 1 mole of sodium sulfate produces 1 mole of sulfate, so 0.500 mol of sodium sulfate produce 0.500 mol of barium sulfate. That said, we have moles of barium sulfate produced. To find mass, let's multiply this amount of moles by the molar mass of barium sulfate:
