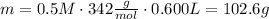Answer:
102.6 g
Step-by-step explanation:
Firstly, let's understand the terms used in the question. Semi-molar solution is a solution which has a molarity of:
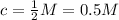
We're given the molar mass of:
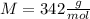
Let's use the definition of molarity: molarity is the ratio between the moles of solute and the volume of solution:
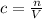
From here, we wish to express moles, n, as the ratio of mass of sucrose to its molar mass:
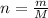
Substitute it back into the equation of molarity:
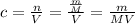
Since we wish to find mass, let's multiply both sides of the equation by MV to obtain mass equation:
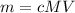
Now, convert volume into liters knowing that 1 mL = 1 cm³ and 1000 mL = 1 L:
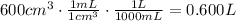
Substitute all three variables into the equation: