Answer:
The precipitate will form.
Step-by-step explanation:
Let's write the equilibrium expression for the solubility product of calcium sulfate:
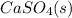 ⇄
⇄
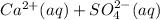
The solubility product is defined as the product of the free ions raised to the power of their coefficients, in this case:
![K_(sp)=[Ca^(2+)][SO_4^(2-)]=10^(-4.5)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/2agxuok64t99kqwhb7o8scij80jqqmx7uk.png)
Our idea is to find the solubility quotient, Q, and compare it to the K value. A precipitate will only form if Q > K. If Q < K, the precipitate won't form. In this case:
![Q_(sp)=[Ca^(2+)][SO_4^(2-)]=5.00\cdot10^(-2) M\cdot7.00\cdot10^(-3) M=3.5\cdot10^(-4)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/yfjk7nvkwtkqcapwac29wf1u7lye3immij.png)
Now given the K value of:
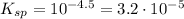
Notice that:
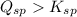
This means the precipitate will form, as we have an excess of free ions and the equilibrium will shift towards the formation of a precipitate to decrease the amount of free ions.