Answer:
Pressure in acetylene gas tank will be 74.8atm
Step-by-step explanation:
Step 1: Using the ideal gas equation, determine the number of moles of oxygen
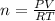
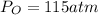
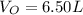
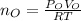
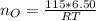
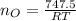
As temperature is unknown and assumed to be the same for both gases, and the ideal gas constant will be the same for both cases, these values are left as constants 'T' and 'R'.
Step 2: Determine the proportionate number of moles of acetylene required based on the chemical equation
2 moles of acetylene require 5 moles of oxygen for complete combustion
Thus, 0.4 moles of acetylene are required per mole of oxygen
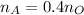
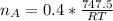
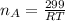
Step 3: Determine the pressure of acetylene tank required in 4.00L tank
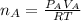
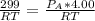

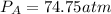
Assumptions:
Temperature is the same for both gases and constant
The ideal gas constant is the same for both gases
The combustion reaction is complete and there are no limiting factors