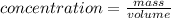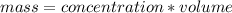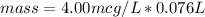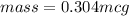Answer:
Maximum allowable mass of cadmium in a 76.0mL sample is 0.304μg (0.304mcg)
Step-by-step explanation:
In the complete question, the limit for cadmium is defined as 4.00μg/L.
μg can also be denoted as mcg
The concentration of cadmium is the amount of mass of cadmium per unit volume of water. The limit for the mass of cadmium in the measured sample can be found by multiplying the volume of water by the allowed concentration.