Answer:
Hydrogen is the limiting reagent.
Step-by-step explanation:
Limiting Reagent : It is the substance that get consumed when the reaction complete. It is used to determine the what amount of product will be formed.
When
 and
and
 react, it will produce
react, it will produce
 gas . The balance reaction can be written as:\
gas . The balance reaction can be written as:\
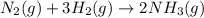
Here, 1 mol
 require 3 mol
require 3 mol

In question ,
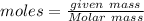
Molar mass of H2 = 2 g/mol , given mass = 5 g
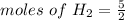
 = 2.5 mole
= 2.5 mole
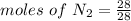
Molar mass of N2 = 28 g/mol , given mass = 28 g
 = 1 mole
= 1 mole
Since, 1 mole
 require 3 mole
require 3 mole

But available , mole of
 = 2.5
= 2.5
So we need extra 0.5 moles of Hydrogen gas
Hence
 get consumed first , Thus Hydrogen is the limiting reagent
get consumed first , Thus Hydrogen is the limiting reagent