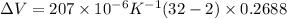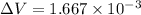Answer:
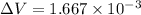
Step-by-step explanation:
Given the initial temperature T_i=2° C
final temperature T_f= 32° C
The original volume of water Vo=268.8 mL= 0.2688 L
we need to calculate the change in the volume
As we know that volume expansion is given by
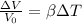
ΔV= change in Volume
β= expansion coefficient =
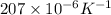
therefore,
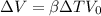
plugging values we get