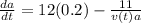Answer:
differential equation fora in terms of t is
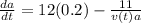
Step-by-step explanation:
Given :
Rate at which the reaction takes place is proportional to the square root of the amount of substance A present.
the amount of substance A present (measured in grams) at any time t = a
orbitary constant = k
Solution
The rate of change of the reaction is
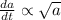
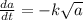
Thus , if amount present is low then
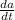 will be high
will be high
Now
Volume = v(t) = 25-(12-11)t
Volume v(t) = 25 –t
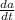 = 12 (200 grams) - 11/(v(t))a
= 12 (200 grams) - 11/(v(t))a
converting 200 gram to kg we get
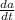 = 12 (0.2) - 11/(v(t))a
= 12 (0.2) - 11/(v(t))a