Answer:
The answer to your question is 25.9 g of KCl
Step-by-step explanation:
Data
Grams of KCl = ?
Volume = 0.75 l
Molarity = 1 M
Formula
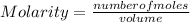
Solve for number of moles
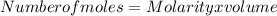
Substitution
Number of moles = 1 x 0.75
Simplification
Number of moles = 0.75 moles
Molecular mass KCl = 39 + 35.5 = 34.5
Use proportions to find the grams of KCl
34.5 g of KCl ---------------- 1 mol
x ---------------- 0.75 moles
x = (0.75 x 34.5) / 1
x = 25.9 g of KCl