Answer:
Daughter nucleus
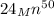
Step-by-step explanation:
A nuclear reaction undergoes positron emission, or sometimes called beta plus decay, when a proton of a radioactive nuclei converts into a neutron and thereby emits a positron and an electron neutrino in the process. A removal of proton and addition of neutron after positron emission results to a decrease in the atomic number of the nucleus while unaffecting its mass number.