Answer:
the mass of the lipid content, to the nearest hundredth of a kg, in this solution =0.46 kg
Step-by-step explanation:
Total heat content of the fat = heat content of water +heat content of the lipids
Let it be Q
the Q= (mcΔT)_lipids + (mcΔT)_water
total mass of fat M= 0.63 Kg
Q= heat supplied = 100 W in 5 minutes
ΔT= 20°C
c_lipid= 1700J/(kgoC)
c_water= 4200J/(kgoC)
then,
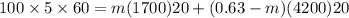
solving the above equation we get
m= 0.46 kg
the mass of the lipid content, to the nearest hundredth of a kg, in this solution =0.46 kg