Answer:
Vibrational Energy Of HCl in the lowest state :
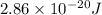
Classical Limit for stretching of HCl bond from its equilibrium length :
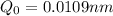
Percent of equilibrium Bond Length :
8.58 %
Step-by-step explanation:
H-Cl bond Length = 0.127 nm =
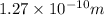
Frequency from v = 0 to v = 1 is 2886
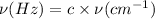
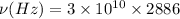
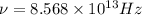
Reduced mass
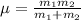
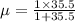
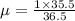
but this has units in amu , to convert it in Kg divide it by
 and 1000(to convert gram itno Kg)
and 1000(to convert gram itno Kg)
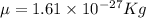
Calculation of Force constant :
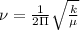
Here,
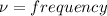
k = force constant
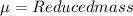
Put the value of frequency , reduced mass and calculate for force constant
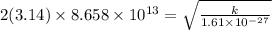
Solve the left hand side and square it. Then multiply it with reduced mass
k = 475.97 N/m
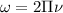
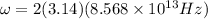
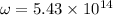
Calculation of lowest energy
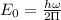
h = planck's constant =
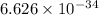
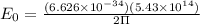
On solving ,
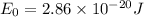
Calculation of Stretching of HCl bond:
Use formula :
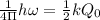
here Q = stretching bond length
Put , k = 475.97 N/m ,
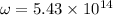 and solve for Q
and solve for Q
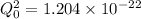
take square root

Calculation of Percentage extension:
Percentage
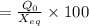
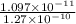
Percentage = 8.58 %