Answer:
The electronengativity values of given elements is as follows.
Fluorine - 4
Chlorine -3
Bromine - 2.9
Iodine- 2.5
Step-by-step explanation:
Electronegativity =consant (I.E-E.A)
The electron affinity and ionization energy values of the given elements is as follows.
(In attachment)
First we have to find the value of constant by using the fluorine atom to whom the electronengativity taken as "4".
Fluorine:
![4=constant[1678-(-327.8)]](https://img.qammunity.org/2020/formulas/chemistry/college/e6qwecr1ixpn8zkdmb7vsg3zbh1dhuqqvc.png)
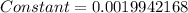
By using this constant values we can find electronegatvity values of remaining elements.
Chlorine:
![Electronegativity=0.0019942168[1255+348.7]=3.1980\sim 3](https://img.qammunity.org/2020/formulas/chemistry/college/me7nn0oer2gno5hvo6uvz0tihc7i9tmez2.png)
Therefore, electronegativity of chlorine is 3.
Bromine:
![Electronegativity=0.0019942168[1138+324.5]=2.91\sim 2.9](https://img.qammunity.org/2020/formulas/chemistry/college/jn5oti18drpq7t5obkxut7n93nndtht3wg.png)
Therefore, electronegativity of bromine is 2.9.
Iodine:
![Electronegativity=0.0019942168[1007+295.7]=2.59\sim 2.5](https://img.qammunity.org/2020/formulas/chemistry/college/o50qmu65g207jd8fsc1pupquz08y94bpec.png)
Therefore, electronegativity of iodine is 2.5.