Answer:
The steps are explained below, the essential step is to find mass here, 120 g of NaOH.
Step-by-step explanation:
In order to answer this question, we need to define molarity conceptually firstly to see what variables we need. According to the formula, molarity is equal to the ratio between moles and volume, while moles itself is a ratio between mass and molar mass. This means we have a formula for molarity involving mass, molar mass and volume:
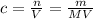
In order to prepare a 500.0 mL of stock solution of 6.0 M of NaOH, we then need to find the mass of NaOH dissolved in this solution using the equation above:
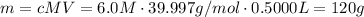
Now, since we have the mass of NaOH, we can describe the steps needed to prepare this solution:
- measure 120 grams of solid NaOH;
- add this mass of NaOH into a 500.0-mL Erlenmeyer flask;
- fill approximately half of the flask with distilled water and stir gently to make sure that NaOH dissolves, if it doesn't, add more water and repeat the process;
- when NaOH fully dissolves, fill the flask to the mark.
Our solution is prepared.