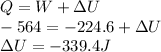Answer:
Part 1)
- 224.6 J
Part 2)
- 339.4 J
Step-by-step explanation:
 = Constant pressure acting on gas = 0.643 atm = (0.643) (1.013 x 10⁵) Pa = 0.651 x 10⁵ Pa
= Constant pressure acting on gas = 0.643 atm = (0.643) (1.013 x 10⁵) Pa = 0.651 x 10⁵ Pa
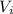 = initial volume = 5.46 L = 0.00546 m³
= initial volume = 5.46 L = 0.00546 m³
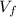 = final volume = 2.01 L = 0.00201 m³
= final volume = 2.01 L = 0.00201 m³
Work done on the gas is given as
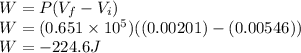
Part 2)
 = Change in the internal energy
= Change in the internal energy
 = Heat energy escaped = - 564 J
= Heat energy escaped = - 564 J
Using First law of thermodynamics