Answer:
4.8 Liters.
Explanation:
When pressure is kept constant the volume of a certain quantity of gas is proportional to the absolute temperature of the gas.
So,
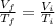 .......... (1)
.......... (1)
Where, f denotes the final and i denotes the initial values.
Now,
 Liters,
Liters,
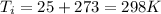 and
and
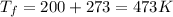
Therefore, from equation (1) we get,
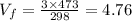 Liters ≈ 4.8 Liters. (Answer)
Liters ≈ 4.8 Liters. (Answer)