Answer:
2.1 × 10⁻¹⁹ M/s
Step-by-step explanation:
Let's consider the degradation of CF₃CH₂F by OH radicals.
CF₃CH₂F + OH → CF₃CHF + H₂O
Considering the order of reaction for each reactant is 1 and the rate constant is 1.6 × 10⁸ M⁻¹s⁻¹, the rate law is:
r = 1.6 × 10⁸ M⁻¹s⁻¹.[CF₃CH₂F].[OH]
where,
r is the rate of the reaction
If the tropospheric concentrations of OH and CF₃CH₂F are 8.1 × 10⁵ and 6.3 × 10⁸ molecules/cm³, respectively, what is the rate of reaction at this temperature in M/s?
The Avogadro's number is 6.02 × 10²³ molecules/mole.
The molar concentration of OH is:
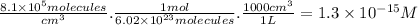
The molar concentration of CF₃CH₂F is:
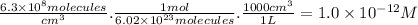
r = 1.6 × 10⁸ M⁻¹s⁻¹ × 1.0 × 10⁻¹² M × 1.3 × 10⁻¹⁵ M = 2.1 × 10⁻¹⁹ M/s