Answer
given,
distance between the nuclei of an O₂ molecule = 1.20 x 10⁻¹⁰ m
mass of oxygen atom = 2.66 x 10⁻²⁶ Kg
the reduced mass of O₂ molecule =
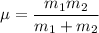
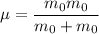
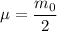
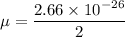

moment of inertia of O₂ molecule
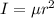
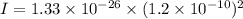
I = 1.9152 x 10⁻⁴⁶ kg.m²
a) Rotational energy of oxygen molecule
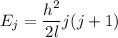
J = 0
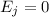
J = 1
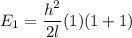
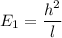

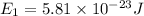
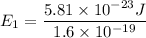
E₁ = 3.63 x 10⁻⁴ eV
J = 2
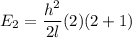
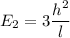
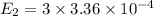
E₂ = 1.089 x 10⁻³ eV
b) Effective force constant between the molecule
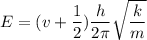
for v = 0
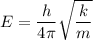
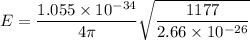
E = 1.569 x 10⁻²¹ J
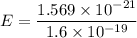
E₀ = 9.8 x 10⁻³ eV
for v = 1
E₁ = 3 E₀
E₁ = 3 x 9.8 x 10⁻³
E₁ = 29.4 x 10⁻³ eV
For v = 2
E₂ = 5 E₀
E₂ = 5 x 9.8 x 10⁻³
E₂ = 49 x 10⁻³ eV