Answer:
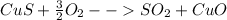
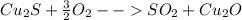
Step-by-step explanation:
Hello,
At first, for the covellite, CuS, the balanced chemical reaction turns out into:
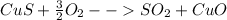
Secondly, for the chalcocite, Cu2S, the balanced chemical reaction turns out into:
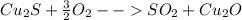
Such reactions are quite the same in terms of the stoichiometric coefficient of oxygen.
Best regards.