Answer:
Moles of boron trifluoride gas that were collected = 11.6 mol
Mass of boron trifluoride gas that were collected = 787 g
Step-by-step explanation:
Given that:
Temperature = 2.0 °C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T = (2.0 + 273.15) K = 275.15 K
V = 15.0 L
Pressure = 0.130 atm
Using ideal gas equation as:

where,
P is the pressure
V is the volume
n is the number of moles
T is the temperature
R is Gas constant having value = 0.0821 L atm/ K mol
Applying the equation as:
0.130 atm × 15.0 L = n ×0.0821 L atm/ K mol × 275.15 K
⇒n = 11.6 mol
Thus, Moles of boron trifluoride gas that were collected = 11.6 mol
Molar mass of boron trifluoride gas = 67.82 g/mol
The formula for the calculation of moles is shown below:
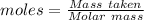
Thus,
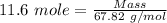
Mass of boron trifluoride gas that were collected = 787 g