Answer:
(a) -0.00017 M/s;
(b) 0.00034 M/s
Step-by-step explanation:
(a) Rate of a reaction is defined as change in molarity in a unit time, that is:
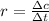
Given the following reaction:
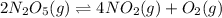
We may write the rate expression in terms of reactants firstly. Since reactants are decreasing in molarity, we're adding a negative sign. Similarly, if we wish to look at the overall reaction rate, we need to divide by stoichiometric coefficients:
![r = -(\Delta [N_2O_5])/(2 \Delta t)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/phe3oldihj0gsjh5cdda7o0bla5m6bfrhe.png)
Reaction rate is also equal to the rate of formation of products divided by their coefficients:
![r = (\Delta [NO_2])/(4 \Delta t) = (\Delta [O_2])/(\Delta t)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/5437r8x4w13y7lz1yxmd7dtxscle3bcn7w.png)
Let's find the rate of disappearance of the reactant firstly. This would be found dividing the change in molarity by the change in time:
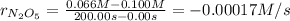
(b) Using the relationship derived previously, we know that:
![-(\Delta [N_2O_5])/(2 \Delta t) = (\Delta [NO_2])/(4 \Delta t)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/n54ba26l0atxuz58boyrs94zobwk51bvwt.png)
Rate of appearance of nitrogen dioxide is given by:
![r_(NO_2) = (\Delta [NO_2])/(\Delta t)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/tyrsdeae5jmigosztd537aao4ha0sqjf49.png)
Which is obtained from the equation:
![-(\Delta [N_2O_5])/(2 \Delta t) = (\Delta [NO_2])/(4 \Delta t)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/n54ba26l0atxuz58boyrs94zobwk51bvwt.png)
If we multiply both sides by 4, that is:
![-(4 \Delta [N_2O_5])/(2 \Delta t) = (\Delta [NO_2])/(\Delta t)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/jnop1xjaxytkdq4c2jmusz51zfo8d2ngqw.png)
This yields:
[tex]r_{NO_2} = \frac{\Delta [NO_2]}{\Delta t} = -2\frac{\Delta [N_2O_5]}{ \Delta t} = -2\cdot (-0.00017 M/s) = 0.00034 M/s[tex]