Step-by-step explanation:
Initial mass of the isotope 90-Sr in baby= 1.00 μg
Formula used :
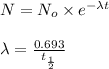
where,
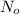 = initial mass of isotope
= initial mass of isotope
N = mass of the parent isotope left after the time, (t)
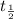 = half life of the isotope = 28.1 years
= half life of the isotope = 28.1 years
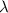 = rate constant
= rate constant
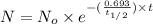
a) Time taken by the sample, t = 18 years
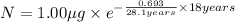
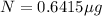
0.6415 μg will remain after 18 years.
b) Time taken by the sample, t = 70 years
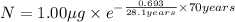
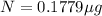
0.1779 μg will remain after 70 years.