Answer:
The differences are listed below.
Step-by-step explanation:
A trivial explanation would be: those are different compounds belonging to different classes (categories) of chemical substances.
Let's look at several differences that these two compounds have:
- calcium carbonate has a chemical formula of
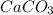 and calcium oxide has a chemical formula of
and calcium oxide has a chemical formula of
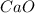 ;
; - calcium carbonate is considered to be a salt, while calcium oxide simply belongs to the class of basic (metallic) oxides;
- calcium carbonate is insoluble in water, it doesn't dissociate in water and doesn't react with it in any other way. In contrast, calcium oxide reacts with water to produce calcium hydroxide;
- calcium carbonate has a much lower melting point compared to calcium oxide. This is due to the fact that in CaO, calcium is bonded to a very small oxygen anion as opposed to a bulky carbonate anion in calcium carbonate. This yields a much greater Coulombic attraction force in CaO and a much higher melting point.
- another difference would simply be the fact that calcium carbonate consists of 3 different elements, calcium, carbon and oxygen, while CaO only consists of 2 different elements, calcium and oxygen.