Answer:
Free energy change of the reaction is +98.83 kJ/mol.
Step-by-step explanation:
The dissociation reaction of mercury(I) aqueous solution is as follows.
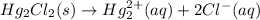
The standard free energy of the reaction is as follows.
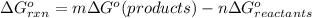
![=[(1* \Delta G^(o)_{f(Hg_(2)^(2+))})+(2* \Delta G^(o)_(f(Cl^(-))))-(1* \Delta G^(o)_{f(Hg_(2)Cl_(2))})]](https://img.qammunity.org/2020/formulas/chemistry/college/sf2dz6tnsiu1zehd6zaq7nxduq6gz8p82k.png)
![=[(1* 153.5+2* -131.25)-(1* -210.78)]kJ=+101.78kJ](https://img.qammunity.org/2020/formulas/chemistry/college/l7lx0bgkbudj2ackzr5puvhs4l8ljnxf4z.png)
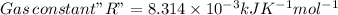
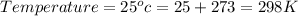
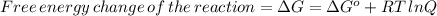
![\Delta G=\Delta G^(o)+RT\,ln([Hg_(2)^(2+)][Cl^(-)]^(2))](https://img.qammunity.org/2020/formulas/chemistry/college/5cv95bcr4dtmf43frz1z33hj5oe9moqswq.png)
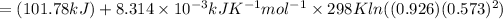
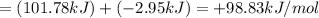
Therefore, Free energy change of the reaction is +98.83 kJ/mol.