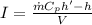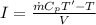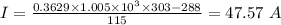Answer:
The amount of current supplied to heater is 47.57 A
Solution:
As per the question:
Voltage of electric heater, V = 115 V
Volume rate of flow of air,
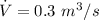
Initial temperature of air, T =
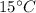 = 273 + 15 = 288 K
= 273 + 15 = 288 K
Final temperature of air, T' =
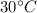 = 273 + 30 = 303 K
= 273 + 30 = 303 K
Initial Pressure = Final Pressure, P = 100 kPa
Specific heat of air,
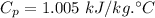
Gas constant, R = 0.287
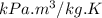
Now,
To compute the amount of current supplied to the heater, we assume the steady state operation and neglecting the effects of kinetic energy and potential energy:
Specific volume, 'v' of the air at the inlet is given by:
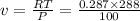
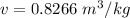
Mass flow rate of air can be given by:
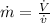
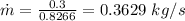
Considering the system of the pipe containing air and using the energy balance on this system:
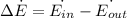
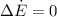
Therefore,
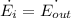
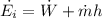
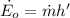
Thus
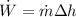
Also, we know that:
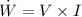
where
I = current in amperes
Now, comparing the two eqns, we get: