Answer:
The pressure is changing at
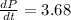
Explanation:
Suppose we have two quantities, which are connected to each other and both changing with time. A related rate problem is a problem in which we know the rate of change of one of the quantities and want to find the rate of change of the other quantity.
We know that the volume is decreasing at the rate of
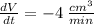 and we want to find at what rate is the pressure changing.
and we want to find at what rate is the pressure changing.
The equation that model this situation is
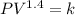
Differentiate both sides with respect to time t.
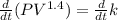
The Product rule tells us how to differentiate expressions that are the product of two other, more basic, expressions:
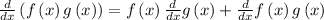
Apply this rule to our expression we get
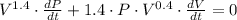
Solve for
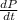
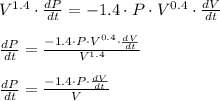
when P = 23 kg/cm2, V = 35 cm3, and
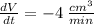 this becomes
this becomes
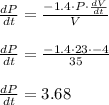
The pressure is changing at
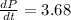 .
.