Answer:
The coefficient of
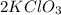 is 2
is 2
Step-by-step explanation:
The Balanced Chemical equation is :
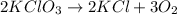
This reaction is balanced because total number of atoms of different elements in reactant is equal to total number of atoms in products.
Reactant : 2
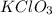
Number of K atoms = (2)1 = 2
Number of Cl atoms = (2)1 = 2
Number of O atoms = (2)3 = 6
Product : 2 KCl and 3

In KCl
Number of K atoms = (2)1 = 2
Number of Cl atoms = (2)1 = 2
In
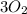
Number of O atoms = (3)2 = 6
So ,
this equation is balanced and follow Law of Conservation of Mass because :
Number of K atom in reactant = product = 2
Number of Cl atom in reactant = product = 2
Number of O atom in reactant = product = 6