Answer:
0.567 kJ
Step-by-step explanation:
In the given problem, 50.0 mL of 0.400 M HBr at 24.35°C is added to 50.0 mL of 0.400 M NaOH, also at 24.35°C. After the reaction, the final temperature is 27.06°C. To calculate the enthalpy change per mole of HBr (in kJ), we have:
The balanced chemical equation for the reaction is shown below.
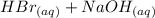 ⇒
⇒
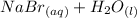
We also need to calculate the number of moles of the reactants in the system.
For the system above, the enthalpy change is:
ΔH = heat capacity * temperature change = 4.184 J/°C * (27.06 - 24.35) °C = 11.339 J
number of moles (n) = concentration (c) *volume (v)
For HBr, n = 0.05*0.4 = 0.02 moles
For NaOH, n = 0.05*0.4 = 0.02 moles
Thus, to calculate the enthalpy change by mole, we have:
11.339 J/0.02 moles = 566.93 J
Therefore, the enthalpy change per mole of HBr (in kJ) is 0.567 kJ