Answer:
We will collect 11.17 liters of oxygen gas.
Step-by-step explanation:
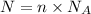
Where:
N = Number of particles / atoms/ molecules
n = Number of moles
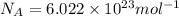 = Avogadro's number
= Avogadro's number
We have:
Molecules of oxygen gas N =
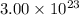
n =?
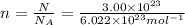
n = 0.4982 moles of oxygen
Using ideal gas equation:
PV = nRT
where,
P = Pressure of gas = 1 atm (at STP)
V = Volume of gas = 10 L
n = number of moles of gas = ?
R = Gas constant = 0.0821 L.atm/mol.K
T = Temperature of gas = 273.15 K (at STP)
Putting values in above equation, we get:
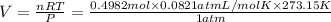
V = 11.17 L
We will collect 11.17 liters of oxygen gas.