Answer:
The molar concentration of the original Cu²⁺ solution: M₁ = 0.196 M
Step-by-step explanation:
Given: Reaction 1: Dilution-
Original Cu²⁺ solution: Volume: V₁ = 10.0 mL, Molarity: M₁=?
Diluted Cu²⁺ solution: Volume: V₂ = 75 mL, Molarity: M₂ = ?
Reaction 2: Titration of Diluted Cu²⁺ solution with-
Na₂S₂O₃ solution: Volume: V₃ = 13.05 mL, Molarity: M₃ = 0.15 M
The overall reaction involved is:
2Cu²⁺ + 2Na₂S₂O₃+ 4KI → 2CuI + 4K⁺ + 2NaI + Na₂S₄O₆
In this titration reaction, equal moles of Cu²⁺ and Na₂S₂O₃ reacts.
So to find the concentration of diluted Cu²⁺ solution (M₂), we use the equation:
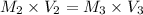
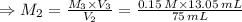
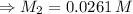
Now, to find the concentration of the original Cu²⁺ solution (M₁), we use the equation:
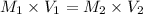
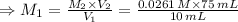
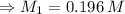
Therefore, the molar concentration of the original Cu²⁺ solution: M₁ = 0.196 M