Answer:
Step-by-step explanation:
Initial moles of Acetic acid=
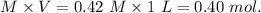
Initial moles of potassium acetate =
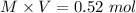
since, CaOH is a strong base. So, after its addition i react with acetic acid.
Moles of acetic acid left=
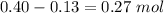
Moles of potassium acetate=

Now, we check all given options:
A. False ( because it gets consumed by KOH)
B. True
C. False ( it decrease because of addition of base)
D. False ( It increase because of addition of base)
E. False ( Because con of
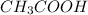 decrease and
decrease and
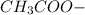 increase.
increase.