Answer:
D
Explanation:
H+ is the hydrogen ion concentration.
This problem requires the use of solving log equation.
PH of vinegar is 2.9, lets find the number of hydrogen ions in it by using the equation:
![PH=-Log[H^(+)]\\2.9=-Log[H^+]\\-2.9=Log_(10)[H^+]](https://img.qammunity.org/2020/formulas/mathematics/middle-school/xz5yjex8x8jj8hd06m0phd89bc3do14pfh.png)
Note: Log is normally base 10 if not specified
Now, to solve, we use exponential to log conversion formula shown below:
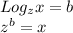
Now lets solve:
![-2.9=Log_(10)[H^+]\\H^+=10^(-2.9)\\H^+=0.001259](https://img.qammunity.org/2020/formulas/mathematics/middle-school/fjexnpy0aod4dmnjiqpe9028gixi4qiarz.png)
Now, the PH of milk is 6.6, so we follow same procedure and find the number of hydrogen ion in milk. Shown below:
![PH=-Log[H^(+)]\\6.6=-Log[H^+]\\-6.6=Log_(10)[H^+]\\H^+=10^(-6.6)\\H^+=0.0000002512](https://img.qammunity.org/2020/formulas/mathematics/middle-school/7ua9idlq1fjrwjuqn8t6mm2ymyt2q969rt.png)
So, to find how many times it is greater, we divide first answer we found by the second answer:
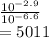
That is about 5000 times, answer D is right.