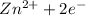Answer:
(a) The anode electrode which comprises the zinc electrode being placed in a water solution with low oxygen concentration.
(b) Cathodic reaction is:
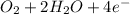 ⇒
⇒
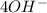
Anodic reaction is:
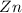 ⇒
⇒
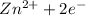
Step-by-step explanation:
In the given problem, we have an oxygen-concentration cell consisting of two zinc electrodes. One is immersed in a water solution with a low oxygen concentration and the other in a water solution with a high oxygen concentration. The zinc electrodes are connected by an external copper wire.
(a) Which electrode will corrode?
The electrode that will corrode is the anode electrode which comprises the zinc electrode being placed in a water solution with low oxygen concentration.
(b) Write half-cell reactions for the anodic reaction and the cathodic reaction.
Cathodic reaction is:
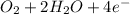 ⇒
⇒
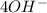
Anodic reaction is:
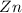 ⇒
⇒