Answer:
An increase in air temperature because of its compression.
Step-by-step explanation:
The Gay-Lussac's Law states that a gas pressure is directly proportional to its temperature in an enclosed system to constant volume.
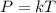
where P: is the gas pressure, T: is the gas temperature and k: is a constant.
Therefore, due to Gay-Lussac's Law, when the plunger is pushed down very rapidly, the pressure of the air increase, which leads to its temperature increase. That is why cotton flashes and burns.
I hope it helps you!