Answer:
The mass of silver nitrate is 555 grams.
Step-by-step explanation:
The given salt is silver nitrate.
The solubility refers to the amount of a given solute to dissolve into the solvent at different temperature and pressure.
The solubility of silver nitrate at different temperatures is as follows.
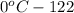
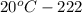

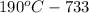
The mass of silver nitrate can be dissolved
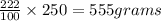
Therefore, The mass of silver nitrate is 555 grams.