Answer:
Fluorine
Step-by-step explanation:
Remembering the equation for Coulombic attraction, we know that an electrostatic attraction between two charges is directly proportional to the product of the two charges and inversely proportional to the square of a distance between them. This is modeled by the equation:
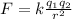
The attraction force we are analyzing in this problem occurs between a nucleus of a halogen and its valence electrons. Essentially, according to the equation, the greater the distance between a nucleus and valence electrons, the lower the attraction force. This implies that a valence electron would be lost more easily from an atom which has the highest radius.
Going down the group, the number of electron shells increases and the radius of halogens increases. This means that fluorine has the smallest radius and attracts its valence electrons strongest.