Answer:
q = - 2067.2 J of Heat is giving out when 85.0g of lead cools from 200.0 c to 10.0 c.
Step-by-step explanation:
The Specific Heat capacity of Lead is 0.128
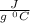
This means, increase in temperature of 1 gm of lead by
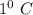 will require 0.128 J of heat.
will require 0.128 J of heat.
Formula Used :
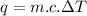
q = amount of heat added / removed
m = mass of substance in grams = 85.0 g
c = specific heat of the substance = 0.128
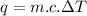 = Change in temperature
= Change in temperature
= final temperature - Initial temperature
= 10 - 200
= -
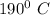
put value in formula
q = -
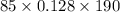
On calculation,
q = - 2067.2 J
- sign indicates that the heat is released in the process