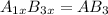Step-by-step explanation:
In a hexagonal-close-pack (HCP) unit cell, the ratio of lattice points to octahedral holes to tetrahedral holes = 1 : 1 : 2
let the :
Number of lattice point = 1x.
Number of octahedral points = 1x
Number of tetrahedral points = 2x
If anions occupy the HCP lattice points and cations occupy half of the octahedral holes.
Number of anions occupying the HCP lattice points, A= 1x
Number of cations occupying the octahedral points, B = 1x
The formula of the compound will be =
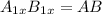
If anions occupy the HCP lattice points and cations occupy all of the octahedral and the tetrahedral holes.
Number of anions occupying the HCP lattice points, A= 1x
Number of cations occupying the octahedral points, B = x
Number of cations occupying the tetrahedral points, B = 2x
total number of cations = x + 2x = 3x
The formula of the compound will be =