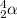Answer:
Step-by-step explanation:
In this question, we wish to find the missing nuclei for the equation:
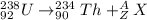
In order to find the missing species, we need to use the charge and mass balance law. That is, the mass should be conserved: the total mass on the left-hand side with respect to the arrow should be equal to the total mass on the right-hand side with respect to the arrow:
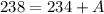
Notice from here that:
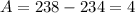
So far we know that the mass of X is 4. Similarly, we apply the law of charge conservation. The total charge should be conserved:
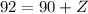
From here:
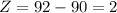
We have a particle:
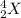
Looking at the periodic table, an atom with Z = 2 corresponds to helium. This can also be written as an alpha particle: