Answer:
32,0 days.
Step-by-step explanation:
The radioactive decay follows:
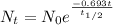
Where Nt is the concentration in a time t (6,22x10¹⁷), N₀ is the initial concentration (9,95x10¹⁸) Half life time is 8,0 days and t is the time it take to drop this concentration. Replacing:
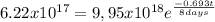
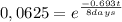
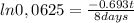
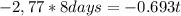
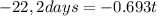
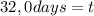
It take 32,0 days
I hope it helps!