Answer:
The percent yield is 42.52%.
Step-by-step explanation:
Theoretical yield of beryllium chloride = 12.7 g
Actual yield of the beryllium chloride = 5.4 g
The formula used for the percent yield will be :
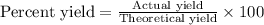
Now put all the given values in this formula, we get:
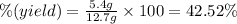
The percent yield is 42.52%.