Answer:
a)n =7 , b) n = 5
Step-by-step explanation:
The energy levels of the hydrogen atom is described by the Bohr model
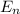 = - 13.606 1/n²
= - 13.606 1/n²
This equation energy is given in elector volts and n is an integer
A transition occurs when the electro sees from a superior to a lower state
E₀ -
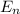 = -13.606 (1/
= -13.606 (1/
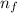 ² - 1/n₀²)
² - 1/n₀²)
Let's apply this expression
n₀ = 2
Let's look for the energy of the different levels and subtract it
n₀
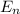 (eV)
(eV)
1 -13,606
2 -3.4015
3 - 1.5118
4 -0.850375
5 -0.54424
6 -0.3779
7 -0.2777
The wavelength of the transition is 397 nm = 397 10⁻⁹ m
The speed of light is related to wavelength and frequency
c = λ f
The Planck equation gives the energy of a transition
E = h f
E = h c /λ
Let's calculate
E = 6.63 10⁻³⁴ 3 10⁸/397 10⁻⁹
E = 5.01 10⁻¹⁹ J
Let's reduce to eV
E = 5.01 10⁻¹⁹ J (1 eV / 1.6 10⁻¹⁹ J)
E = 3.1313 eV
Let's examine the possible transitions from the initial level ni = 2
ΔE =
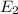 -
-
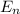 = -3.1313
= -3.1313
En = 3.13 -3.4015
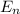 = 0.2702 eV
= 0.2702 eV
When examining the table we see that the level that this energy has is the level of n = 7
Part B the transition is in the infrared
The frequency is 74 10¹² Hz
We use the Planck equation
E = h f
E = 6.63 10⁻³⁴ 74 10¹²
E = 4.9062 10⁻²⁰ J
E = 4.9062 10⁻²⁰ / 1.6 10⁻¹⁹
E = 0.3066 eV
We look for the level with the energy difference
ΔE = E₄-
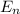 = 0.3066
= 0.3066
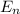 = 0.3066 - 0.85037
= 0.3066 - 0.85037
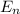 = -0.54376 eV
= -0.54376 eV
When examining the table this energy has the level n = 5, therefore from this level the transition occurs