Answer:
A) 277 million years
Explanation:
Here, decay of U-238 is first order reaction. (since most of the nuclear decays are first order).
so, the equation t =
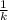 × ln(
× ln(
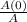 )
)
where, t = time from start of reaction
k = reaction constant
A(0) = initial concentration; A = present concentration.
⇒ 4.5 billion = 4500 million years =
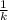 × ln2.
× ln2.
Now, given 95.82% of parent U-238 isotope is left.
⇒ t =
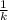 × ln(
× ln(
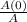 )
)
t =
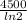 × ln(
× ln(
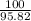 )
)
⇒ t ≅ 277 million years.