Answer:
The correct answer is option A.
Step-by-step explanation:
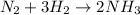
Moles of nitrogen gas =
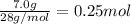
According to reaction, 1 mole of nitrogen reacts with 3 moles of hydrogen gas.
Then 0.25 moles of nitrogen gas will react with:
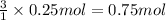 of hydrogen gas.
of hydrogen gas.
Mass of 0.75 moles of hydrogen gas = 0.75 mol × 2 g/mol = 1.5 g
1.5 grams of hydrogen that would be required to completely react with this amount of nitrogen.